Bulbitech
Every eye movement matters
Bulbitech empowers healthcare providers with advanced eye movement platform for identifying and applying functional biomarkers invisible to conventional methods.
MDR Class IIa certified and software-driven, Bulbitech’s mission is to enable earlier, more effective, and objective detection of neurological and ophthalmological conditions.
BulbiCARE®
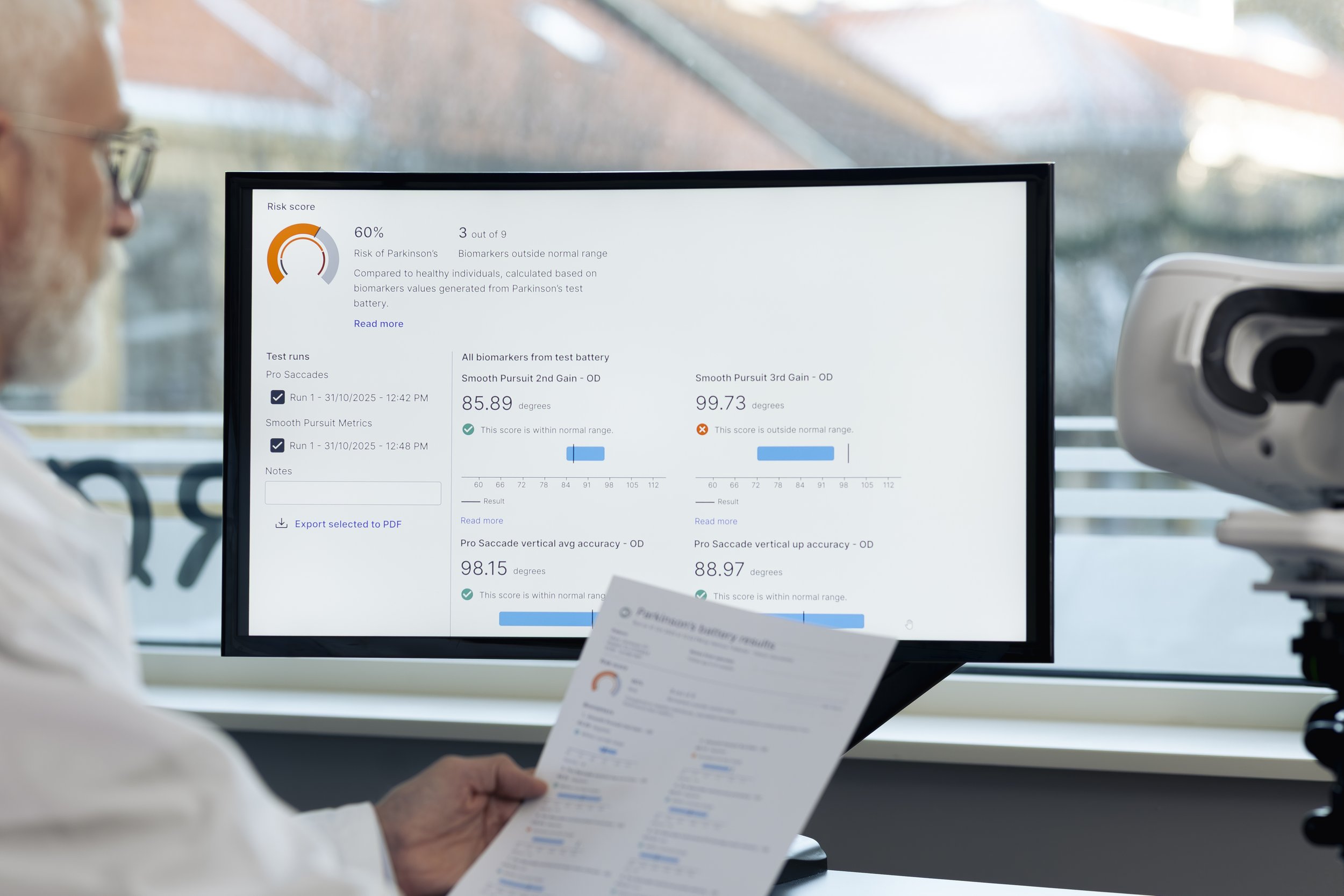
Objective Data When You Need Clarity
Quantified eye movement analysis that complements traditional examination methods.
Validated biomarkers, consistent results, simple workflow.

Latest news
Research Partners