Next-generation diagnostics with eye-tracking
Our Clinical Importance
Early detection of functional disorders with a dedicated platform and diagnostic device that replaces the manual examination and saves time.
Decentralizing access to screening of brain function by introducing rapid test based on eye tracking.
Rapid, automated screening of brain function supports already understaffed neurological staff by providing them with diagnostic support platform in which the patient does the work and can be operated by anyone.
Generating objective brain function data and results to improve patient lives, health outcome and support research.
Current Tests
Our Purpose
Our aim is to offer clinicians and researchers one device that can perform multiple rapid neuro-ophthalmic eye examination tests. Our unique BulbiHUB platform and BulbiCAM currently offers the user multiple tests to screen for glaucoma, age-related macular degeneration (AMD) , nystagmus, cataract and optic neuritis. Ultimately our BulbiCAM will include multiple tests to screen for both ophthalmological and neurological conditions.
Our technology maintains good vision and cognitive health across a lifetime.
BulbiCAM - Multiple rapid eye examination tests to screen for ophthalmological and neurological conditions
Benefits of Our Technology
- Rapid testing the afferent visual function and efferent system of patients
- One combined perimeter, pupillograph and nystagmograph
- Early detection of ophthalmological and neurological conditions
- Provides objective data of the afferent visual function and efferent system of patients
- Replaces manual examinations
- Patient does the work, not the clinician
- Integrates with any third party ophthalmic device
- Can be operated by ophthalmic nurse, tech staff or any other assistant
Problems We Solve
- Long and difficult process of neuro-ophthalmic examination
- Manually performing the entrance and functional eye examination
- Manually inserting test results
- Inefficient clinical workflow
- Subjective interpretation
- Lack of data during clinical decision making
- Lack of research data on eye - brain functionality
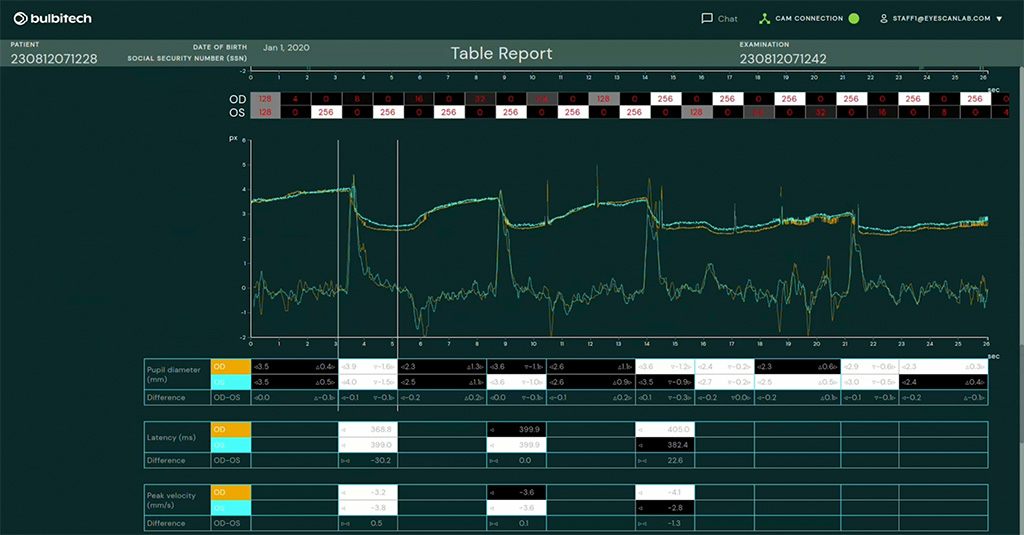
BulbiCAM
Diagnostic tool in mastering a complete neuro-ophthalmic assessment of the patient

- 400-600 FPS eyetracking
- Two screens for independent presentation to each eye
- Accurate timing synchronization
- Calibration free eye tracking test setup
- Individual compensation of refractive errors and head shape
- Can be operated remotely via internet
- CE-certified & FDA registered
Our Solution
BulbiHUB Platform
BulbiHUB platform assesses the data measured by BulbiCAM. BulbiHUB is an innovative point-of-care eye platform for the screening of ophthalmological and neurological disorders.
Telemedicine
Assessed data can be shared between and amongst clinical from peripheral locations to one central location, resulting in a clinical workflow that can continue remotely during pandemic outbreaks
AI [Artificial Intelligence]
Algorithms can be developed to detect early stage neuro- ocular dysfunctions and analyze our collected data for research purpose
News
Meet Bulbitech at FLORetina-ICOOR 2024
Bulbitech is attending the FLORetina-ICOOR Meeting 2024 (Floretina ICOOR 2024) from December 5th to 8th at the Firenze Fiera convention center in […]
Join Bulbitech at the NHS Research Scotland Ophthalmology and Scottish Eye Research and Innovation Meeting on 22nd November
Exciting news for anyone passionate about advancing ophthalmology! Join Bulbitech at the NHS Research Scotland Ophthalmology and Scottish Eye Research and Innovation […]
Dr. Jimmy Uddin and Dr. Mohsan Malik win at Innovate Thyroid Eye Disease 2024 Symposium
Innovative Research on Thyroid Eye Disease In a notable achievement at the Innovate Thyroid Eye Disease (TED) 2024 Multidisciplinary Symposium, Dr. Jimmy […]
Clinical validation at Oslo University Hospital demonstrates the reliability and stability of BulbiCAM® technology and identifies valuable biomarkers for Parkinson’s disease
Bulbitech and Meddoc Medical Research Institute, Norway, in collaboration with the Neurological Department at Oslo University Hospital, will soon release promising results […]
Business Norway published an article about our technology
Bulbitech revolutionises eye and neurological disease diagnostics and care. Bulbitech delivers AI-powered eye-tracking technology that screens for ophthalmological and neurological disorders. “Our […]
Digital Health’s Rewired 2024 Pitchfest Heaths
We’re proud to announce we’ve been shortlisted as one of the 15 companies of the Digital Health’s Rewired 2024 Pitchfest Heaths. Looking […]
Investor news
Bulbitech has raised 5,7 MNOK in an emission and 4,8 MNOK in convertible loans as part of a bridge round with existing […]
Bulbitech’s newest team member Richard J. Connors
Bulbitech is delighted to inform you that we are welcoming Richard John Connors to our team as part of the MSCA Doctoral […]
Pupil 2.0 test video review
Pupil test video review. During the examination, and while fixating on the red target, each eye is exposed to a screen of […]
New studies confirm the BulbiCAM Pupil test’s reliability and precision in eye and brain health
We are excited to share with you groundbreaking studies that offer a comprehensive evaluation of BulbiCAM, our leading diagnostic tool. In a […]
Our Company
Our Timeline
2015
Funding from public funds and private investors for technology development
2016
Technical R&D development on platform & hardware
2017
Company & software CE-certified
2018
Funding from public funds and private investors to complete smaller prototype.
Certified acc to ISO 13485:2016
2019
Finalist in the DNB Healthcare prize
2020
Bulbitech wins healthcare pitching contest at Oslo Innovation Week
Hardware CE-certified
2021
Bulbitech is FDA registered under number D437315
Awarded € 2 million euro grant from Norwegian Research Council for clinical validation of tests
2022
Semifinalist EIT Health Catapult 2022-2023.
Certified acc to 27001:2013
2023
Completed two validation studies in neurology (Parkinson’s & Ataxia)

I find the BulbiCAM device to be fascinating and shaping the future leading trends in ophthalmology. It is simple and easy to use, modular and very ergonomic. With its advanced virtual reality technology and diagnostic and monitoring abilities for eye disorders, this will likely be a breakthrough technology in the daily practice of high turnover clinics.
Prof. Goran Petrovski
Department of Ophthalmology, Oslo University Hospital and University of Oslo, Norway, The opinions stated by Prof. Goran Petrovski are his own, and not related to the institution he works at
I am impressed by the technology of your device and first of all I would like to congratulate you for trying to solve a combination of clinical problems that no device can reliably solve.